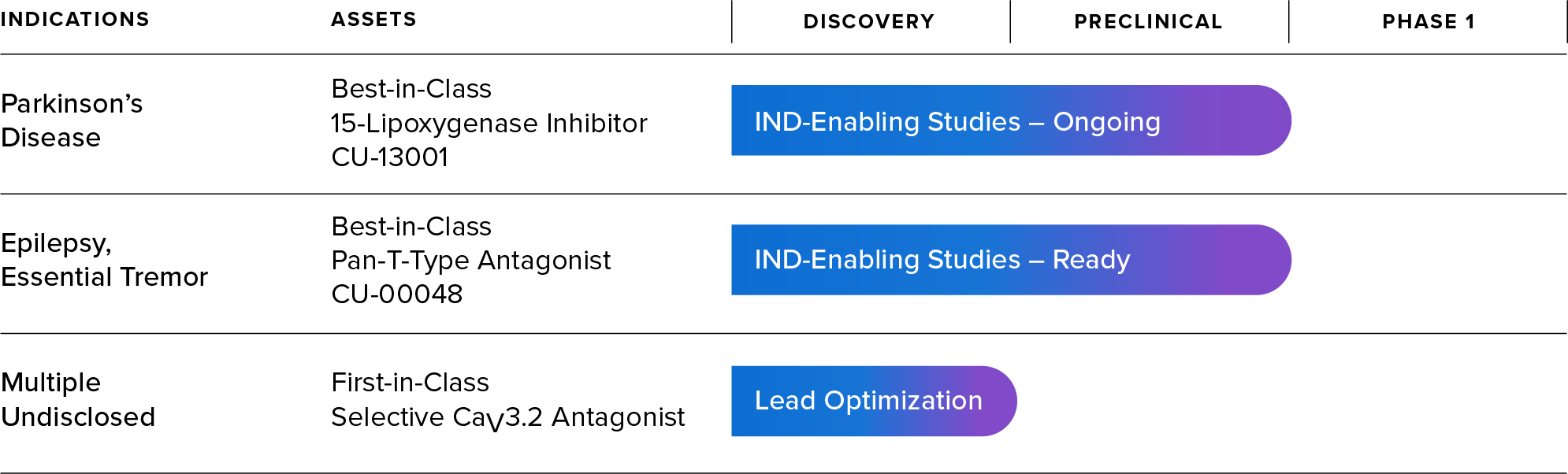
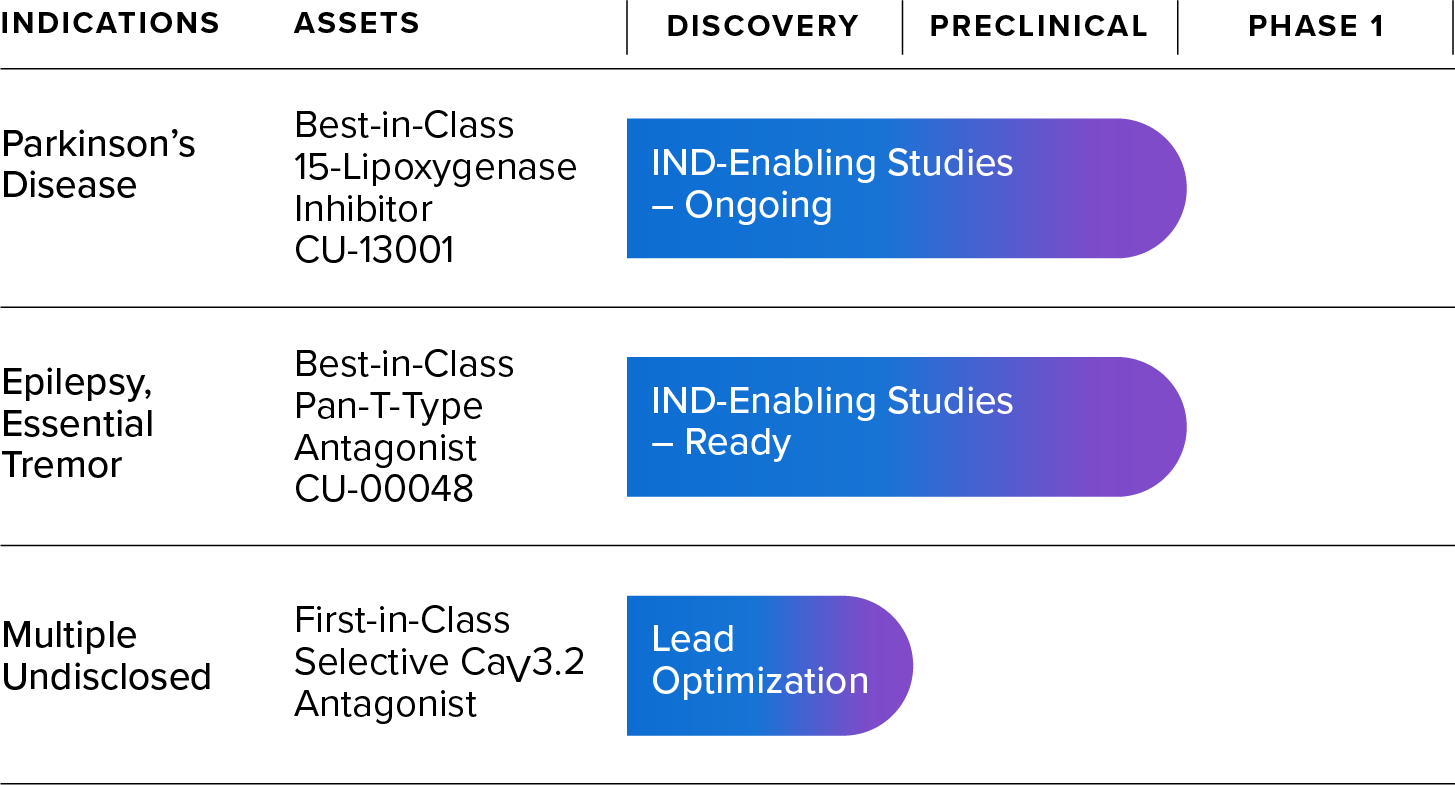
Our Pipeline

Therapeutic Programs
15-LO Inhibitor
Our lead therapeutic candidate, CU-13001, is designed to inhibit 15-lipoxygenase (15-LO). In preclinical studies, by inhibiting 15-LO, CU-13001 significantly reduces the production of 4-HNE, thus reducing LRRK2 kinase hyperactivity and α-synuclein aggregation in both genetic and idiopathic forms of PD.
Support for the role of this pathway in PD pathology was recently published in Science Translational Medicine1. Additional publications and the filing of an Investigational New Drug (IND) application for CU-13001 are expected in 2026. This will allow us to begin clinical studies in healthy adult volunteers, followed rapidly by studies in PD patients.

Selective CaV3.2 Antagonist
Acurex has discovered a first-in-class selective antagonist of the CaV3.2 voltage-gated calcium channel that can serve as a starting point for finding a lead drug candidate. This channel plays a significant role in Parkinson’s disease and other pathologies by dysregulating calcium balance in the cell2,3. In PD, calcium dysregulation leads to mitochondrial stress, neuroinflammation, and dopaminergic deficits, ultimately resulting in neurodegeneration. Due to the wide-ranging impact of CaV3.2 in neurons, this program may offer symptomatic relief and potentially slow or stop neurological disease pathologies. Acurex plans to optimize a lead drug candidate over the next several months for eventual preclinical and clinical development.

T-Type Calcium Channel Inhibitor
Voltage-gated calcium channels have been targets of interest for CNS indications due to their role as essential regulators of neural communication4–8. T-type calcium channels are the only low-voltage activated calcium channels and play a key role regulating calcium oscillations and neuronal excitability. Acurex has developed a best-in-class pan T-type antagonist, CU-00048, that is highly selective to T-type calcium channels, has excellent drug-like properties, and is ready for IND-enabling studies. Acurex plans to partner this program for further development.
References
- Keeney, M. T. et al. LRRK2 regulates production of reactive oxygen species in cell and animal models of Parkinson’s disease. Sci Transl Med 16, 17–20 https://doi.org/10.1126/scitranslmed.adl3438 (2024).
- 2. Gaare, J. J. et al. Rare genetic variation in mitochondrial pathways influences the risk for Parkinson’s disease. Mov Disord 33, 1591–1600 https://doi.org/10.1002/mds.64 (2018).
- Tabata, Y. et al. T-type Calcium Channels Determine the Vulnerability of Dopaminergic Neurons to Mitochondrial Stress in Familial Parkinson Disease. Stem Cell Reports 11, 1171–1184 https://doi.org/10.1016/j.stemcr.2018.09.006 (2018).
- Weiss, N. & Zamponi, G. W. T-type calcium channels: From molecule to therapeutic opportunities. Int J Biochem Cell Biol 108, 34–39 https://doi.org/10.1016/j.biocel.2019.01.008 (2019).
- Kopecky, B. J., Liang, R. & Bao, J. T-type calcium channel blockers as neuroprotective agents. Pflugers Arch 466, 757–65 https://doi.org/10.1007/s00424-014-1454-x (2014).
- Cain, S. M. & Snutch, T. P. Contributions of T-type calcium channel isoforms to neuronal firing. Channels 4, 475–482 https://doi.org/10.4161/chan.4.6.14106 (2010).
- Matthews, L. G. et al. T-type calcium channels as therapeutic targets in essential tremor and Parkinson’s disease. Ann Clin Transl Neurol 10, 462–483 https://doi.org/10.1002/acn3.51735 (2023).
- Iftinca, M. C. & Zamponi, G. W. Regulation of neuronal T-type calcium channels. Trends Pharmacol Sci 30, 32–40 https://doi.org/10.1016/j.tips.2008.10.004 (2009).
